New High-powered Microscopes for Stem Cell Research
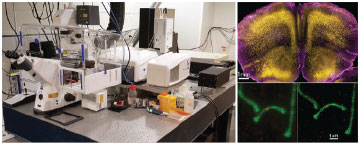
Cornell has led the development of advanced optical microscopy and fluorescence-based techniques for biomedical research since the 1970s. In this tradition, Prof. Warren Zipfel (BME) and Dr. Rebecca Williams (Director of the Biotechnology Resource Center (BRC) Imaging Facility) recently secured more than $2.5 million from various sources (NIH, NSF, and NYS) to purchase three user-friendly, high-end instruments that are now part of the BRC Imaging Facility and the New York State Stem Cell Optical Imaging Core. Installed in 2015, these new instruments include two combined confocal/multiphoton microscopes and a super-resolution instrument capable of resolving objects as small as 20 nm. Many of the technologies now part of these commercial instruments, such as multiphoton microscopy, were originally developed at Cornell. Along with the other light microscopes, a fluorescence cell sorter, micro- and nano-CT instruments, and the high-resolution ultrasound technologies available at the imaging facility, Cornell researchers across campus now have access to a complete set of modern imaging and fluorescence analysis tools.
Cornell’s reputation as a microscopy powerhouse started with Watt Webb’s group (Applied & Engineering Physics) who pioneered fluorescence recovery after photobleaching (FRAP) and fluorescence correlation spectroscopy (FCS). The invention of two-photon (or multiphoton) microscopy in 1990 by the same group resulted in a new form of 3D micrometer-scale microscopy that enables deep imaging, even in living samples. Prof. Webb is now Emeritus, but many alums of his lab remain at Cornell. Zipfel, previously a researcher with Webb, is an associate professor in BME and Williams, who got her Ph.D. under Webb, is an adjunct professor in BME as well as the BRC Imaging Facility Director. Zipfel and Williams both helped develop and commercialize multiphoton microscopy in the 1990s. In the mid-2000s, as a new class of fluorescence microscopy known as super-resolution microscopy emerged, the Zipfel lab built an instrument based on this technique. These types of light microscopes are called “super-resolution” because they can acquire images that surpass the resolution limit, known as the diffraction limit, of a conventional microscope. With a conventional microscope, any object smaller than 250 nm still looks like a 250-nm-wide spot, but super-resolution microscopes can improve resolution by two- to ten-times.
In 2006 Zipfel and Williams moved to BME, merging their microscopy development center with a new incarnation of the BRC Imaging Facility. The majority of the lab instruments were home-built, therefore providing custom functionality for specific tasks, but they were often not very user-friendly. The BRC facility had two relatively new confocal microscopes, but its multiphoton microscope was a two-decades-old confocal microscope converted to multiphoton functionality by the Zipfel lab in 2000. Today, with the new acquisitions, the BRC is a Life Sciences Resource Center that, in addition to the latest advances in imaging, runs core facilities for genomics, proteomics, genomic diversity, bioinformatics, and bio-computing.

